Selected Tufts CTSI Community Health Catalyst Program projects will be funded for one year, beginning May 1, 2021 and ending April 30, 2022, pending NCATS approval.
Institutional and Regulatory Approvals
Project start and release of funds are contingent upon receipt of all necessary local institutional and regulatory approvals. While such approvals are not required at the time of proposal submission, award recipients will be expected to initiate the approval application process upon receiving their notice of award. If applicable, proof of exempt status determination must be obtained. All applicable local regulatory approvals and/or determination letters must be received by no later than April 2021.
NCATS Prior Approval Requirement
In addition to receiving local institutional and regulatory approvals, projects involving human subjects, as determined by the IRB, are required to comply with requirements for the NCATS Human Subject Research Prior Approval. To ensure all requirements are met by the award start date, both applicants and award recipients are strongly encouraged to carefully review the NCATS guidelines and discuss any potential concerns with Tufts CTSI Community Health Catalyst Program staff ahead of time.
- Human Subjects Research: Projects involving human subjects are required to comply with requirements for the NCATS Human Subject Research Prior Approval. As of April 9, 2020, award recipients conducting human subjects research, including research that is otherwise exempt under Title 45 Part 46 of the Code of Federal Regulations, will be required to submit their IRB approvals and any applicable NCATS-required documentation to Tufts CTSI before their projects can begin. Additionally, those whose research is deemed to be greater than minimal risk, meet the criteria for an NIH-defined clinical trial, or include a foreign component may not begin their projects until NCATS official prior approval for their projects is received. Generally, the NCATS human subjects research prior approval process takes 30 days. However, if a request is returned for any reason and if approval is sought for a clinical trial, the turnaround time may take up to 60 days.
- Non-human Subjects Research: Projects that do not involve human subjects are not required to seek NCATS prior approval. However, if requested, award recipients will be required to provide proof of exclusion status determination issued by the IRB.
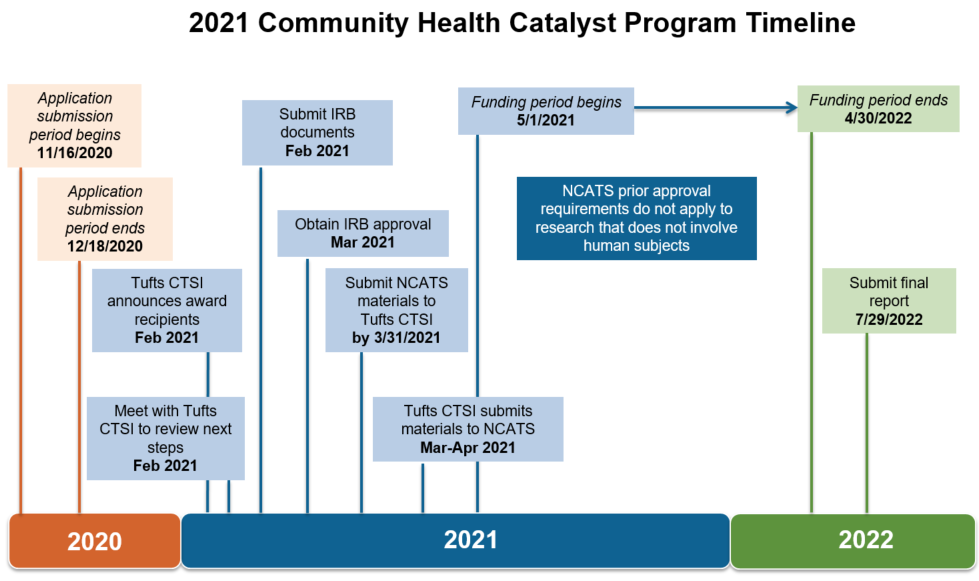
Description of 2021 Community Health Catalyst Timeline.
Questions?
Please review our Frequently Asked Questions.
Need further assistance? We are here to help. Please contact us at community@tuftsctsi.org.



