Request for Applications
The Tufts Clinical and Translational Science Institute (CTSI) Community Health Catalyst Program seeks proposals for collaborative, community-driven translational research projects undertaken jointly by community-based organizations and investigators from Tufts CTSI-affiliated partner organizations.
The Community Health Catalyst Program is not currently accepting applications.
Key Dates
- Application submission period begins: Monday, November 16, 2020
- Application submission period ends: Friday, December 18, 2020 at 11:59 PM
- Award announcement: February 2021
- Award period begins: Saturday, May 1, 2021
- Award period ends: Saturday, April 30, 2022
- Final project reports due: Friday, July 29, 2022
*Special Considerations for 2021*
All applicants will be asked to outline how they propose to complete the proposed research within the award period despite possible COVID-19-related closures and/or associated restrictions on research activities.
Award Information
About the Program
The Community Health Catalyst Program is a funding opportunity supported by the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH). Launched in 2019, the program provides one-year awards to new, developing, and existing community-researcher partnerships that address pressing and overlooked health issues impacting the well-being of communities and the broader public. It supports collaborative, community-driven, translational research projects that leverage the diverse expertise and assets of community-based organizations and Tufts CTSI-affiliated academic or clinical institutions. It prioritizes actionable research that improves health outcomes of the community and advances health equity for traditionally marginalized, underserved, and underrepresented populations.
The program supports community- or investigator-initiated research projects that focus on a specific community-identified need. The proposed studies must be scientifically sound and have the potential to generate meaningful results or knowledge to help advance prevention practices, support development of and access to new treatments and cures for disease, and/or increase the overall effectiveness and quality of health care and public health interventions. They can include, but are not limited to, those that gather preliminary qualitative or quantitative data on a health research question of interest, evaluate an existing health-focused program, or develop a new health intervention. To be considered for funding, applicants should articulate the value and impact of the proposed research project as well as the potential for supporting existing efforts and/or future work. A strong commitment to collaborative work and shared decision-making and an ability to meaningfully engage key stakeholders are essential.
Eligibility Criteria
To be eligible for an award, applications must designate a representative from a community-based organization with tax-exempt status and a Principal Investigator (PI) with a primary appointment or position at a Tufts CTSI-affiliated academic or clinical research organization. Community representatives may or may not be a Tufts CTSI-affiliated community partner or collaborator organization. Proposed studies should be innovative and have no current funding or only modest intramural or extramural support that will be enhanced by Community Health Catalyst Program research project activities.
Award Period and Project Launch Requirements
Selected projects will be funded for one year from May 1, 2021 through April 30, 2022. Project start and release of funds will be contingent upon receipt of all applicable local institutional Review Board (IRB) approvals and/or exclusion determination letters. In addition, projects involving human subjects, as determined by the IRB, will be required to comply with requirements for the NCATS Human Subject Research Prior Approval. More information about project launch requirements is available here.
Award Amounts
Awards will be made to research teams for up to $15,000 per award. Funds can be allocated across sites in accordance with the projects’ needs. They may only be used to cover direct costs that are necessary and reasonable to complete the proposed research project. Such direct costs may include key personnel and support staff (e.g., student or research assistant) salaries and fringe benefits; consultant services; stipends or compensation for stakeholders involved in the project; participant incentives; linguistic translation of consent forms or other project-related materials, and domestic travel, if related to the study. The budget justification should describe the importance of each budgeted item and/or service. The personnel justification should include the name, role, and effort for every budgeted individual on the project. If necessary, applicants may earmark part of their budget for collaborator-supported research activities.
Complementary In-kind Support
In addition to direct funding, award recipients will be provided access to a robust array of complementary research resources and services as well as offered publication support.
- Research Resources and Services: Award recipients will be provided ongoing pre- and post-award scientific and logistical support to help address translational roadblocks that may arise. It will be offered by a support team co-led by Tufts CTSI research-active clinical faculty and staff.
- Clinical and Translational Research Center (CTRC) Support: Applicants and co-applicants with a primary position or faculty appointment at Tufts Medical Center (Tufts MC) or Tufts University (TU) may leverage the Tufts CTSI CTRC Voucher Program for an additional $5,000 of in-kind clinical research services. Eligible applicants may request such support (e.g., study coordination, regulatory assistance, and use of clinical facilities and equipment) as part of their Community Health Catalyst Program application.
- Publication Support: Tufts CTSI will have funding available for journal fees, including publication processing and open access charges. Award recipients may request such support for one eligible publication per research project.
Eligibility Information
Research Team Members
Proposals must designate a representative from a community-based organization with a tax exempt status obtained through the Internal Revenue Service (e.g., 501(c)(3) or other) and a Principal Investigator (PI) with a primary appointment or position at a Tufts CTSI-affiliated academic or clinical organization. Unless agreed upon in advance, the PI’s institution will be expected to serve as the “lead” or “prime” on the project. Research teams may include other collaborators, provided their involvement is necessary for the conduct of the project.
Eligible Lead Sites
Academic Partners and Collaborators
- Brandeis University
- Massachusetts General Hospital Institute of Health Professions
- Massachusetts Institute of Technology
- Northeastern University
- RAND Corporation
- The Jackson Laboratory
- Tufts University
Clinical Partners and Collaborators
- Baystate Medical Center
- Lahey Hospital and Medical Center
- Maine Medical Center
- New England Baptist Hospital
- Newton-Wellesley Hospital
- Elizabeth’s Medical Center
- Tufts Medical Center
Foreign Component
Applicants proposing research involving a foreign component, as defined by NIH, are eligible to apply. However, they must provide a compelling justification for such research and/or involvement of a foreign component (e.g., performing a specific element or segment of a project outside of the U.S., with assistance of a collaborator employed by a foreign entity or a non-U.S. vendor, and/or with financial support or resources from a foreign entity). Furthermore, if selected, they will be required to receive the NCATS prior approval, whether or not funding is requested to support activities to be performed at a foreign site and/or by a foreign entity.
Returning Applicants
Past Tufts CTSI award recipients are welcome to re-apply to obtain funding for a new research project, but will not be considered for additional funding for a project previously awarded through the program. To be considered for new funding, returning applicants must comply with all prior award reporting requirements.
Application Process
Available Pre-award Resources and Services
Applicants are strongly encouraged, but not required, to discuss their proposed projects with Tufts CTSI during the application development phase. Our experts can assist applicants with meeting all eligibility and funding requirements and guide them through the application submission process. They can also provide methodological and technical support, recommend additional project team members, assist teams with project planning, and help identify and involve relevant stakeholders. To speak to a member of Tufts CTSI prior to or during the proposal development phase, please request a consultation or email the Community Health Catalyst Program team at community@tuftsctsi.org.
Applicants are also encouraged to review The Community Members’ Guide to Submitting a Community-Engaged Research Federal Grant Application (PDF). Written specifically for community-based organizations, this guide provides information about community-engaged research, community-academic research partnerships, and community-engaged research application process. Learn more about the guide and access links to additional instructions and resources here.
How to Apply
The Community Health Catalyst Program has a one-step application process. Applications must be submitted here by no later than Friday, December 18, 2020. Incomplete and late submissions will not be accepted.
- Application: The application consists of fields to be filled in online and three program-specific forms. Prior to submission, applicants should access the Tufts CTSI REDCap online platform to download the forms provided by Tufts CTSI. Once the required documents are completed, they should be uploaded as part of the application.
- Letter(s) of Support: While not required, applicants are strongly encouraged to submit up to five letters of support. Letters should come from individuals who are familiar with the specific proposal, team of investigators, and available resources to be provided by participating institutions. They can reinforce institutional support and communicate enthusiasm for the proposed project. It is strongly recommended to customize each letter to the specific signatory and/or to highlight a different strong point.
Application Review Process and Criteria
All complete applications will be reviewed for scientific merit, research significance, and alignment with the program’s overall objectives. They will be rated by two scientific reviewers from Tufts CTSI-affiliated partner organizations and two Tufts CTSI Stakeholder Expert Panel representatives using the 9-point rating scale (1=exceptional; 9 =poor). The review process will follow NIH scoring guidelines based on the criteria listed below.
- Scientific Review
- Relevance– ability to meet NCATS and Tufts CTSI objectives by demonstrating explicit relevance to improved health.
- Significance– quality and merit of the proposed research project.
- Innovation– potential for impact through development of novel solutions and processes.
- Investigators– qualifications of investigators to carry out the proposed research project.
- Environment– availability of resources to support the proposed research project.
- Approach– scientific rigor of the clinical or methodological design plan to meet the proposed objectives and goals.
- Future Plans– clear articulation of next steps for future research, dissemination of project results, and seeking future funding.
- Stakeholder Review
-
- Research team – depth of stakeholder engagement and strength of collaborative researcher/community partnership.
- Relevance–ability to demonstrate explicit relevance and value to community health.
- Approach– rigor of the project design plan to meet proposed objectives and goals.
Funding Decision and Announcement
Final funding decision will be made independently by the Tufts CTSI Senior Leadership Team based on the reviewers’ recommendations, the overall impact score, project feasibility (and/or adaptability to account for possible COVID-19-related closures and/or associated restrictions on research activities), clear strategy and intentional focus on health equity, budget justification, and available funds. Awards will be made only to projects that are scientifically rigorous, can demonstrate effective engagement of relevant stakeholders, and are responsive to the needs of the identified communities. All applicants will be informed of the outcome of their submission via email. Reviewers’ comments will be provided to all primary applicants, regardless of whether or not they are awarded funding.
Confidentiality and Non-disclosure
All proposals will be deemed proprietary and confidential and will be protected against any unauthorized use and any unauthorized or uncontrolled disclosure beyond Tufts CTSI, the Community Health Catalyst Program Review Committee, and the Stakeholder Expert Panel.
Submission Components: From Application to Project Launch
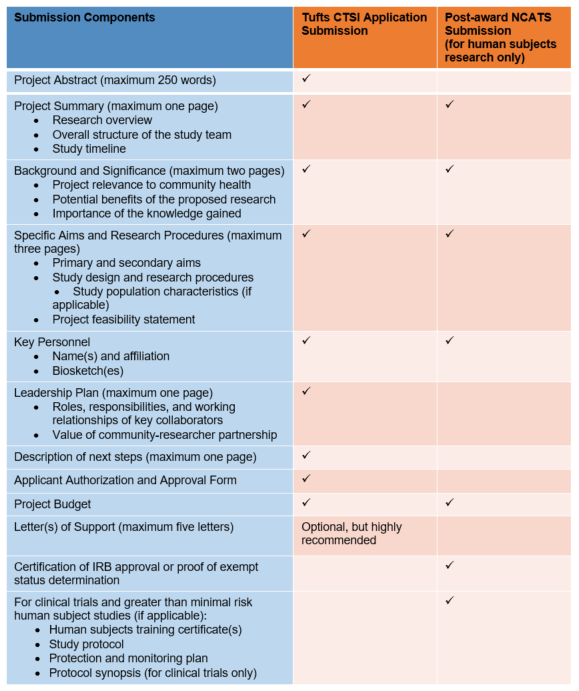
Description of submission components table.
Questions?
Please review our Frequently Asked Questions.
Need further assistance? We are here to help. Please contact us at community@tuftsctsi.org.
Aviva Must, PhD, Director of the Community Health Catalyst Program
Nadia Prokofieva, MA, Senior Project Manager



